Abstract
Background High-risk (HR) newly diagnosed multiple myeloma (NDMM) has poor outcomes with standard first-line therapy, even in transplant-eligible (TE) patients (pts). A CAR-T therapy with high efficacy and manageable safety profile would be a potential solution to this significant unmet need. In a phase 1 multicenter single-arm study GC012F, an autologous B cell maturation antigen (BCMA) and CD19 dual-targeting CAR-T cells therapy developed on the novel FasTCAR-T enabling next-day manufacturing platform [J Clin Oncol 40, 2022 (suppl; abstr 8005) and HemaSphere, 2022;6:(S3):180], showed deep and durable responses with a favorable safety profile in heavily pretreated pts with relapsed or refractory multiple myeloma (RRMM). Based on these promising results, we assessed the safety and feasibility of GC012F CAR-T cell therapy in frontline setting for TE high-risk NDMM pts in a single arm, open-label phase 1 investigator-initiated study (NCT04935580).
Methods TE high-risk NDMM pts were considered eligible for the study if they had one or more of the following features: R-ISS-2 or-3; del17p, t (4;14), t (14;16), or 1q21amp ≥ 4 copies; extramedullary disease (EM); IgD or IgE subtype; LDH > the upper limit of normal; or any of the high-risk definition of mSMART3.0.
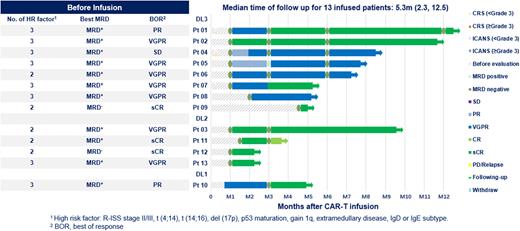
Results of the first 13 pts (median age 59, range 43-65) who received GC012F infusion are reported here, of which the median time from diagnosis to infusion was 4.5 (range 3.2-5.4) months. 100% of the patients had one or more high-risk features including 92.3% R-ISS stage II or III, 69.2% with EM, 33.3% 1q21≥4 copies, and 15.4% IgD type. Twelve pts received 2 cycles induction therapy of bortezomib, lenalidomide and dexamethasone (VRd), and one patient received 1 cycle bortezomib, epirubicin, and dexamethasone (PAD) and 1 cycle VRd prior to the infusion. GC012F was administered as a single infusion at 3 doses levels (DL) of 1x105/kg, 2x105/kg, or 3x105/kg, after receiving a conditioning chemotherapy consisting of cyclophosphamide and fludarabine.Results As of July 25th 2022 data cutoff (the median follow-up 5.3 months, range 2.3-12.5 months), efficacy-evaluable pts (n= 13) was met, with 100% overall response rate (ORR) [95% confidence interval (CI), 72-100)]; 100% of pts (95% CI, 72-100) achieved very good partial response (VGPR) or better, with 69% (95% CI, 39-90) stringent complete response (sCR, Figure 1). 100% (4/4) of pts in DL 2 and 50% (4/8) of pts in DL3 achieved MRD negative-sCR after infusion, which might be the patients in DL2 group carried fewer HR factors than patients in DL3 group. All pts (100%) achieved minimal residual disease (MRD) negativity. There was no difference observed in dose levels. MRD assessment with Euroflow for landmark analysis at 1 month 1 and month 6 post infusion, 100% of evaluable pts were MRD negative at both timepoints. Cytokine release syndrome (CRS) occurred only in three pts (23%) with 15% grade 1 (n=2) and 8% grade 2 (n=1), respectively. There was no treatment-related grade ≥3 CRS and ICANS events. Robust CAR T-cell expansion occurred in all pts with a median time to Tmax of 10 days (range 9-14 d), and peak copy number (Cmax) of 63086 (20097-331159) copies /μg DNA.
Conclusion In this phase I study for transplant-eligible newly diagnosed high-risk MM, BCMA-CD19 dual FasTCAR-T GC012F showed a very favorable safety profile, high efficacy with 100% ORR and 100% MRD negativity. The promising preliminary results warrants further assessment of GC012F for TE NDMM with more patients and longer follow-up.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal